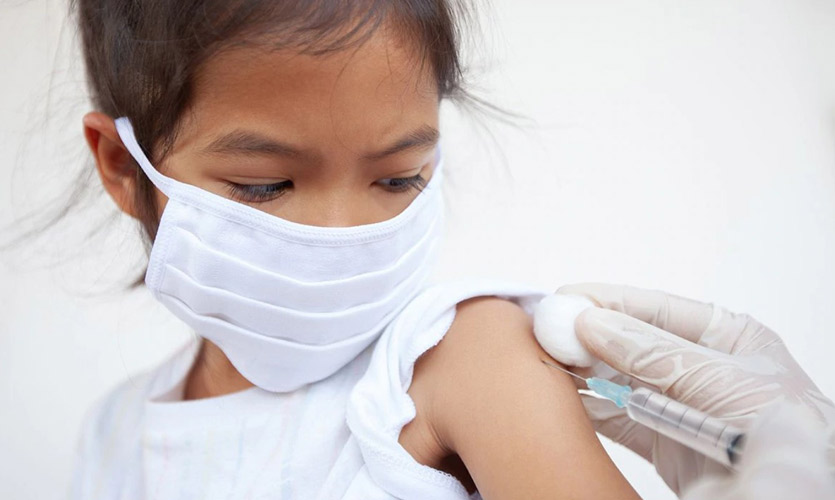
The Drug Controller General of India (DGCI), on Thursday, approved Bharat Biotech’s Covaxin for phase two and three trials on those aged between two and 18 years.
The Hyderabad-based manufacturer said that the trial will be conducted on 525 subjects at various sites including AIIMS in New Delhi and Patna, and the Meditrina Institute of Medical Sciences, Nagpur. As reported, the trial involves two vaccine doses injected on day 0 and day 28.
The COVID-19 Subject Expert Committee (SEC) met on Tuesday to evaluate Bharat Biotech’s application to conduct phase two and three trials “to evaluate the safety, reactogenicity and immunogenicity of Covaxin jabs in children aged 2 to 18 years”.
According to an unnamed source quoted by the Press Trust of India, the committee has imposed a condition that the company should submit interim safety data from the phase 2 clinical study before moving on to phase III.
Bharat Biotech’s Covaxin has been developed in India in association with the Indian Council of Medical Research and the National Institute of Virology. The Oxford-AstraZeneca jab marketed as “Covishield” by the Serum Institute of India (SII) is the other widely used vaccination in India. Russia’s sovereign wealth fund – the Russian Direct Investment Fund (RDIF) – has also acquired regulatory permission in India to manufacture and commercialize its vaccine Sputnik V. Dr. Reddy’s Laboratories will be supposedly managing its vaccine operations in India.
Read more about how the DGCI cleared DRDO’s anti-COVID drug for emergency use.
While various states continue to report COVID-19 vaccine shortages, SII and Bharat Biotech have submitted their production plan for the next four months, Pune-based Serum Institute claims it can increase the output to 10 crore doses by August, while Bharat Biotech claims it can increase the output to 7.8 crore doses.
Covaxin was approved for emergency use last year while still in the clinical trial stage, raising concerns and driving vaccine reluctance in the early phases of India’s immunization program. In a joint statement last month, Bharat Biotech and the ICMR stated that Covaxin was 78% efficient against COVID and functioned against most forms of the virus. They further claimed that the vaccination was completely successful in reducing severe symptoms and hospitalizations.